Difference between revisions of "2723: Outdated Periodic Table"
(→Explanation) |
(→Explanation) |
||
| Line 17: | Line 17: | ||
The conclusion is that Randall's science book was published when those four elements were the only ones in existence, and before the point where practically all the beryllium had decayed. After that point, only the three first would be present, until star formation began and started the process of {{w|Stellar nucleosynthesis}}. | The conclusion is that Randall's science book was published when those four elements were the only ones in existence, and before the point where practically all the beryllium had decayed. After that point, only the three first would be present, until star formation began and started the process of {{w|Stellar nucleosynthesis}}. | ||
| − | Of course no life as we know it could exist until long after stellar nucleosynthesis had created all the other elements needed to support {{w|Carbon-based life}}. And no life, as we could even imagine, would be able to exist for the first 370,000 years after Big Bang as atoms (in a form that could eventually form molecules) could not exist until the {{w|Recombination (cosmology)|Recombination}} phase of the universe, due to the high energy of the {{w|Cosmic background radiation}}. Textbooks, also being | + | Of course no life as we know it could exist until long after stellar nucleosynthesis had created all the other elements needed to support {{w|Carbon-based life}}. And no life, as we could even imagine, would be able to exist for the first 370,000 years after Big Bang as atoms (in a form that could eventually form molecules) could not exist until the {{w|Recombination (cosmology)|Recombination}} phase of the universe, due to the high energy of the {{w|Cosmic background radiation}}. Textbooks, also being carbon-based {{Citation needed}} could not exist either. |
| − | Even now, many {{w|Chemical elements|elements}} do not occur naturally on Earth and have to be {{w|Synthetic element|synthesized}} to be practically studied, or deduced from what was seen during their often short lives. Others were always very hard to detect, collect enough in pure form or purify enough to properly discover them. Until these elements were discovered, one way or another, they were not included in the periodic table. Various versions of the periodic table had left spaces for these {{w|Mendeleev's predicted elements|expected elements}}, but these | + | Even now, many {{w|Chemical elements|elements}} do not occur naturally on Earth and have to be {{w|Synthetic element|synthesized}} to be practically studied, or deduced from what was seen during their often short lives. Others were always very hard to detect, collect enough in pure form or purify enough to properly discover them. Until these elements were discovered, one way or another, they were not included in the periodic table. Various versions of the periodic table had left spaces for these {{w|Mendeleev's predicted elements|expected elements}}, but these gaps have now all been filled, and all recent modifications have been either additions to the end of the prior version of the table or changes of {{w|List of chemical element naming controversies|the names given}} to recent additions. As printed scientific textbooks do not update themselves after being published, one can determine the general age of the work by checking which elements were present in the periodic table that was included. |
| − | The title text refers to how yet-undiscovered elements are given a {{w|systematic element name}} as a temporary name, until a more permanent name is decided upon. The names are based upon a standard group of Greek and Latin roots (depicting the decimal digits used to 'spell out' an element's unique {{w|atomic number}}, i.e. the number of protons) and adding an "-ium" at the end. The claim in the title text is that, in the | + | The title text refers to how yet-undiscovered elements are given a {{w|systematic element name}} as a temporary name, until a more permanent name is decided upon. The names are based upon a standard group of Greek and Latin roots (depicting the decimal digits used to 'spell out' an element's unique {{w|atomic number}}, i.e., the number of protons) and adding an "-ium" at the end. The claim in the title text is that, in the textbook with the figure, researchers claim they have synthesized six additional elements in the second row, temporarily named 'pentium' (atomic number "5") through to 'unnilium' ("one zero", or "10"), just as element "118" was provisionally called "ununoctium". At the time of release of this comic, element 118 is currently the last confirmed element and has been officially called {{w|Oganesson}}. The title text of [[2639: Periodic Table Changes]], the previous comic to draw a periodic table, also refers to this. |
| − | Element number five is, in our time and reality, actually well known as {{w|Boron}}. (Its 'provisional' name of {{w|Pentium}} was also used for a series of microprocessors launched by Intel in the 1990s.) | + | Element number five is, in our time and reality, actually well known as {{w|Boron}}. (Its 'provisional' name of {{w|Pentium}} was also used for a series of microprocessors launched by Intel in the 1990s.) "Unnilium", element number 10, is {{w|Neon}}, a member of the group of {{w|Noble gas}}es, which (along with fellow group member helium) was discovered only at the very end of the 19th century. Despite helium being one of the first elements to exist, and still one of the most common in the universe (roughly 24%, by mass, with hydrogen being around 75% and every other element combined being the remainder), it did not appear in the earliest periodic tables. It was only first detected from afar, as a constituent of the Sun, about thirty years before it was finally physically discovered in an actual lab here on Earth. It is also possible that neon's provisional name in 'early science' might have been something more along the lines of "decium", all else being equal. |
Since life could not have existed at the time this book should have been published, the idea of researchers synthesizing elements, or indeed the existence of books or even researchers, is of course just part of the joke. | Since life could not have existed at the time this book should have been published, the idea of researchers synthesizing elements, or indeed the existence of books or even researchers, is of course just part of the joke. | ||
Revision as of 19:34, 12 January 2023
| Outdated Periodic Table |
 Title text: Researchers claim to have synthesized six additional elements in the second row, temporarily named 'pentium' through 'unnilium'. |
Explanation
| |
This explanation may be incomplete or incorrect: Created by BERYLLIUM-BASED LIFE - Please change this comment when editing this page. Do NOT delete this tag too soon. If you can address this issue, please edit the page! Thanks. |
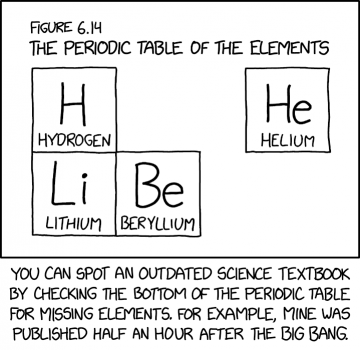
This comic shows figure 6.14 from a science text book, which displays The periodic table of the elements, but with only the first four elements are shown (Hydrogen, Helium, Lithium and Berylium). Randall claims, in the caption, that you can use the layout of an included Periodic table to date a publication based upon the elements present or missing. The joke here is that his book was somehow published just half an hour after the Big Bang, at which time those four elements were the only ones present.
From about 10 seconds until about 20 minutes after the Big Bang, the phase that is known as the Big Bang nucleosynthesis occurred. At that time, hydrogen ions (single protons) provided for helium in abundance and traces of lithium. Some berylium-7 was also formed, which is an unstable isotope, and with a half life of 53 days, an appreciable amount of what had been created would still be there several months after the Big Bang, and certainly most of what was created would be there half an hour after.
The conclusion is that Randall's science book was published when those four elements were the only ones in existence, and before the point where practically all the beryllium had decayed. After that point, only the three first would be present, until star formation began and started the process of Stellar nucleosynthesis.
Of course no life as we know it could exist until long after stellar nucleosynthesis had created all the other elements needed to support Carbon-based life. And no life, as we could even imagine, would be able to exist for the first 370,000 years after Big Bang as atoms (in a form that could eventually form molecules) could not exist until the Recombination phase of the universe, due to the high energy of the Cosmic background radiation. Textbooks, also being carbon-based [citation needed] could not exist either.
Even now, many elements do not occur naturally on Earth and have to be synthesized to be practically studied, or deduced from what was seen during their often short lives. Others were always very hard to detect, collect enough in pure form or purify enough to properly discover them. Until these elements were discovered, one way or another, they were not included in the periodic table. Various versions of the periodic table had left spaces for these expected elements, but these gaps have now all been filled, and all recent modifications have been either additions to the end of the prior version of the table or changes of the names given to recent additions. As printed scientific textbooks do not update themselves after being published, one can determine the general age of the work by checking which elements were present in the periodic table that was included.
The title text refers to how yet-undiscovered elements are given a systematic element name as a temporary name, until a more permanent name is decided upon. The names are based upon a standard group of Greek and Latin roots (depicting the decimal digits used to 'spell out' an element's unique atomic number, i.e., the number of protons) and adding an "-ium" at the end. The claim in the title text is that, in the textbook with the figure, researchers claim they have synthesized six additional elements in the second row, temporarily named 'pentium' (atomic number "5") through to 'unnilium' ("one zero", or "10"), just as element "118" was provisionally called "ununoctium". At the time of release of this comic, element 118 is currently the last confirmed element and has been officially called Oganesson. The title text of 2639: Periodic Table Changes, the previous comic to draw a periodic table, also refers to this.
Element number five is, in our time and reality, actually well known as Boron. (Its 'provisional' name of Pentium was also used for a series of microprocessors launched by Intel in the 1990s.) "Unnilium", element number 10, is Neon, a member of the group of Noble gases, which (along with fellow group member helium) was discovered only at the very end of the 19th century. Despite helium being one of the first elements to exist, and still one of the most common in the universe (roughly 24%, by mass, with hydrogen being around 75% and every other element combined being the remainder), it did not appear in the earliest periodic tables. It was only first detected from afar, as a constituent of the Sun, about thirty years before it was finally physically discovered in an actual lab here on Earth. It is also possible that neon's provisional name in 'early science' might have been something more along the lines of "decium", all else being equal.
Since life could not have existed at the time this book should have been published, the idea of researchers synthesizing elements, or indeed the existence of books or even researchers, is of course just part of the joke.
Transcript
| |
This transcript is incomplete. Please help editing it! Thanks. |
- [Subheading]: Figure 6.14
- [Title]: The periodic table of the elements
- [The following four rectangles featuring the large element abbreviation, with the full element name written below, in a typical periodic table style]
- [Top row, far left]: H Hydrogen
- [Top row, far right, detached from any other box]: He Helium
- [Bottom row, attached directly below the "H" box]: Li Lithium
- [Bottom row, attached directly to the right of "Li"]: Be Beryllium
- [Caption below the panel]:
- You can spot an outdated science textbook by checking the bottom of the periodic table for missing elements. For example, mine was published half an hour after the Big Bang.
Discussion
https://en.wikipedia.org/wiki/Big_Bang_nucleosynthesis BBN did only produce unstable Berylium-7 with a half-life of 53.22 days. Thus after 30 minutes there was still plenty of Be-7 left. --172.71.160.38 15:36, 11 January 2023 (UTC)
"unnilium" is a reference to "unnilunium", which was the name for Mendelevium (atomic number 101; from "un nil un", 1-0-1) before it was given a formal name. Therefore the 6th new element referenced, on top of the 4 already in the table, would be #10, or "un nil", or unnilium. 172.71.190.132
"Pentium" was also the first non-numeric name for the Intel family. Before that, it was the 80486/i486. 172.70.134.195 16:17, 11 January 2023 (UTC)
- Then they chickened out and didn't called the next CPU hexium. -- Hkmaly (talk) 18:24, 12 January 2023 (UTC)
- Maybe they considered the Pro, II, III, 4, etc. just as isotopes (or alternate oxidation states!).
- Though then your criticism still applies to the Core brand (..., 2, i3, etc; although most of that range are clearly imaginary, as well as odd). 162.158.159.75 19:50, 12 January 2023 (UTC)
- Because they didn't want to curse it.172.70.85.200 12:41, 13 January 2023 (UTC)
I have a science textbook at home that doesn't have any elements in a periodic table, though you'd think it should. It has a very nice list of elements (with dodgy details, e.g. I think at least one of them was later proven to be two separate but tricky to isolate elements), but was written prior to the popularisation of Mendeleev's table. (i.e. post-1869, but not by much!) Now, obviously, I don't want to diss Randall's humour, but I have so few such retro-geeky things I can brag about so I just wanted to mention this in passing. (Also, when I actually did my own chemistry, the lab wall had a PT on it that featured the element "Hahniun", and some others since replaced/resolved differently. I sometimes still forget myself and refer to the wrong names if I have to answer trivia questions about them ) 172.70.91.127 23:45, 11 January 2023 (UTC)
- It's a proleptic periodic table Sabik (talk)
- Indeed, but your comment now makes me wonder what a pre-lepton table would look like. ;) 172.70.85.200 10:00, 12 January 2023 (UTC)
Is there a category for comics about the Periodic Table? I recall one from a year or so ago where the symbols were replaced with ones based on the modern names for the elements. Barmar (talk) 05:29, 12 January 2023 (UTC)
- I think you mean 2639:_Periodic_Table_Changes. --172.70.242.156 06:35, 12 January 2023 (UTC)
After an edit, I was left with and adding an -[LINEWRAP HERE]ium at the end, at least on my display (may depend on browser/display width, etc). If I adjusted the to bring the characters away from the line-wrap point then it could just break again whenever/wherever it found itself back in the same bit of the screen again, so I tried to quote it, but got and adding an "-[LINEWRAP HERE]ium" at the end anyway. If anyone can recall how to implement a 'non-breaking non-space' character, or perhaps wishes to aplly a whole wrap-preventing enclosing tag, then I invite them to apply it there. Assuming it isn't edited out of existence, already, and making the issue moot. ;) 172.70.162.222 16:27, 12 January 2023 (UTC)
- Replaced the hyphen with a non-breaking hyphen --172.71.130.54 16:22, 21 February 2023 (UTC)
Would this shape of periodic table (with helium separated from lithium and beryllium) make sense before atoms existed? Would researchers from T+90 minutes even see "periods" as a useful way to organize elements? 172.69.156.159 15:04, 13 January 2023 (UTC)
I wouldn't say no life imaginable could exist half an hour after the Big Bang. Stephen Baxter has written stories with civilizations existing before the inflationary period. --162.158.91.36 07:46, 15 January 2023 (UTC)
This comic was a joke, but this is actually how NCERT textbooks are. The most recent edition is 2006. Link: https://ncert.nic.in/textbook.php
