Difference between revisions of "2723: Outdated Periodic Table"
(→Explanation: element placeholder names are from greek and latin roots) |
(→Explanation: scientists half an hour after the big bang) |
||
| Line 13: | Line 13: | ||
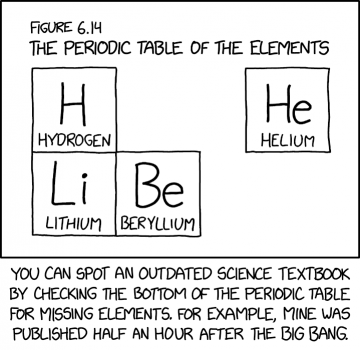
Some {{w|Chemical elements|elements}} do not occur naturally on earth and have to be {{w|Synthetic element|synthesized}}. Those elements are generally excluded from {{w|periodic table|the periodic table}} until they have been synthesized (and are no longer theoretical). At the beginning of the universe, the temperature, and thus the kinetic energy, was too high for matter to form big nuclei. It took some time (how long is not accurately defined) before parts of the universe had cooled down sufficiently for further elements to form naturally. In the first minutes after the Big Bang, only hydrogen, deuterium, helium and lithium nuclei were present. If a scientific textbook had somehow been written at that time (which is highly unlikely, as paper and ink consist of heavier elements such as carbon), it would be able to describe only a very few elements based on observations. | Some {{w|Chemical elements|elements}} do not occur naturally on earth and have to be {{w|Synthetic element|synthesized}}. Those elements are generally excluded from {{w|periodic table|the periodic table}} until they have been synthesized (and are no longer theoretical). At the beginning of the universe, the temperature, and thus the kinetic energy, was too high for matter to form big nuclei. It took some time (how long is not accurately defined) before parts of the universe had cooled down sufficiently for further elements to form naturally. In the first minutes after the Big Bang, only hydrogen, deuterium, helium and lithium nuclei were present. If a scientific textbook had somehow been written at that time (which is highly unlikely, as paper and ink consist of heavier elements such as carbon), it would be able to describe only a very few elements based on observations. | ||
| − | The title text refers to how yet-undiscovered elements are given a {{w|Systematic element name|temporary name according to how many protons they have}}, consisting of Greek and Latin roots. Here the numbers are very low ("pentium" being based on "pent" which means "five") because only four elements had been discovered at the time of publication. {{w|Pentium}} is also the name of a series of microprocessors launched by Intel in the 1990s. | + | The title text refers to how yet-undiscovered elements are given a {{w|Systematic element name|temporary name according to how many protons they have}}, consisting of Greek and Latin roots. Here the numbers are very low ("pentium" being based on "pent" which means "five") because only four elements had been discovered at the time of publication. {{w|Pentium}} is also the name of a series of microprocessors launched by Intel in the 1990s. It also suggests that there were scientists half an hour after the Big Bang who managed to synthesize these heavier elements. However, said scientists probably didn't use the same naming system as us.{{citation needed}} |
==Transcript== | ==Transcript== | ||
Revision as of 21:59, 11 January 2023
| Outdated Periodic Table |
 Title text: Researchers claim to have synthesized six additional elements in the second row, temporarily named 'pentium' through 'unnilium'. |
Explanation
| This is one of 60 incomplete explanations: Created by THE FIRST EKA-HELIUM ATOM - Please change this comment when editing this page. Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
Some elements do not occur naturally on earth and have to be synthesized. Those elements are generally excluded from the periodic table until they have been synthesized (and are no longer theoretical). At the beginning of the universe, the temperature, and thus the kinetic energy, was too high for matter to form big nuclei. It took some time (how long is not accurately defined) before parts of the universe had cooled down sufficiently for further elements to form naturally. In the first minutes after the Big Bang, only hydrogen, deuterium, helium and lithium nuclei were present. If a scientific textbook had somehow been written at that time (which is highly unlikely, as paper and ink consist of heavier elements such as carbon), it would be able to describe only a very few elements based on observations.
The title text refers to how yet-undiscovered elements are given a temporary name according to how many protons they have, consisting of Greek and Latin roots. Here the numbers are very low ("pentium" being based on "pent" which means "five") because only four elements had been discovered at the time of publication. Pentium is also the name of a series of microprocessors launched by Intel in the 1990s. It also suggests that there were scientists half an hour after the Big Bang who managed to synthesize these heavier elements. However, said scientists probably didn't use the same naming system as us.[citation needed]
Transcript
| This is one of 33 incomplete transcripts: Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
- Figure 6.14
- The periodic table of the elements
- H Hydrogen
- He Helium
- Li Lithium
- Be Beryllium
- [Caption below the panel]:
- You can spot an outdated science textbook by checking the bottom of the periodic table for missing elements. For example, mine was published half an hour after the Big Bang.
Discussion
https://en.wikipedia.org/wiki/Big_Bang_nucleosynthesis BBN did only produce unstable Berylium-7 with a half-life of 53.22 days. Thus after 30 minutes there was still plenty of Be-7 left. --172.71.160.38 15:36, 11 January 2023 (UTC)
"unnilium" is a reference to "unnilunium", which was the name for Mendelevium (atomic number 101; from "un nil un", 1-0-1) before it was given a formal name. Therefore the 6th new element referenced, on top of the 4 already in the table, would be #10, or "un nil", or unnilium. 172.71.190.132
"Pentium" was also the first non-numeric name for the Intel family. Before that, it was the 80486/i486. 172.70.134.195 16:17, 11 January 2023 (UTC)
- Then they chickened out and didn't called the next CPU hexium. -- Hkmaly (talk) 18:24, 12 January 2023 (UTC)
- Maybe they considered the Pro, II, III, 4, etc. just as isotopes (or alternate oxidation states!).
- Though then your criticism still applies to the Core brand (..., 2, i3, etc; although most of that range are clearly imaginary, as well as odd). 162.158.159.75 19:50, 12 January 2023 (UTC)
- Because they didn't want to curse it.172.70.85.200 12:41, 13 January 2023 (UTC)
I have a science textbook at home that doesn't have any elements in a periodic table, though you'd think it should. It has a very nice list of elements (with dodgy details, e.g. I think at least one of them was later proven to be two separate but tricky to isolate elements), but was written prior to the popularisation of Mendeleev's table. (i.e. post-1869, but not by much!) Now, obviously, I don't want to diss Randall's humour, but I have so few such retro-geeky things I can brag about so I just wanted to mention this in passing. (Also, when I actually did my own chemistry, the lab wall had a PT on it that featured the element "Hahniun", and some others since replaced/resolved differently. I sometimes still forget myself and refer to the wrong names if I have to answer trivia questions about them ) 172.70.91.127 23:45, 11 January 2023 (UTC)
- It's a proleptic periodic table Sabik (talk)
- Indeed, but your comment now makes me wonder what a pre-lepton table would look like. ;) 172.70.85.200 10:00, 12 January 2023 (UTC)
Is there a category for comics about the Periodic Table? I recall one from a year or so ago where the symbols were replaced with ones based on the modern names for the elements. Barmar (talk) 05:29, 12 January 2023 (UTC)
- I think you mean 2639:_Periodic_Table_Changes. --172.70.242.156 06:35, 12 January 2023 (UTC)
After an edit, I was left with and adding an -[LINEWRAP HERE]ium at the end, at least on my display (may depend on browser/display width, etc). If I adjusted the to bring the characters away from the line-wrap point then it could just break again whenever/wherever it found itself back in the same bit of the screen again, so I tried to quote it, but got and adding an "-[LINEWRAP HERE]ium" at the end anyway. If anyone can recall how to implement a 'non-breaking non-space' character, or perhaps wishes to aplly a whole wrap-preventing enclosing tag, then I invite them to apply it there. Assuming it isn't edited out of existence, already, and making the issue moot. ;) 172.70.162.222 16:27, 12 January 2023 (UTC)
- Replaced the hyphen with a non-breaking hyphen --172.71.130.54 16:22, 21 February 2023 (UTC)
Would this shape of periodic table (with helium separated from lithium and beryllium) make sense before atoms existed? Would researchers from T+90 minutes even see "periods" as a useful way to organize elements? 172.69.156.159 15:04, 13 January 2023 (UTC)
I wouldn't say no life imaginable could exist half an hour after the Big Bang. Stephen Baxter has written stories with civilizations existing before the inflationary period. --162.158.91.36 07:46, 15 January 2023 (UTC)
This comic was a joke, but this is actually how NCERT textbooks are. The most recent edition is 2006. Link: https://ncert.nic.in/textbook.php