Difference between revisions of "3200: Chemical Formula"
(→Explanation) |
(→Explanation) |
||
| (38 intermediate revisions by 13 users not shown) | |||
| Line 11: | Line 11: | ||
==Explanation== | ==Explanation== | ||
{{incomplete|This page was created by the carbon in the universe . Don't remove this notice too soon.}} | {{incomplete|This page was created by the carbon in the universe . Don't remove this notice too soon.}} | ||
| − | + | This supposed "{{w|chemical formula}} for the universe" merely lists the numbers of atoms of each element that are [https://ptable.com/#Properties/Abundance/Universe thought to exist] in the observable universe. Usually, chemical formulae imply rather more of a discrete binding together of the atoms involved. They also represent a single {{w|molecule}} of the substance, rather than trying to cover the entire number of atoms in the whole quantity under consideration. | |
| + | |||
| + | This may be poking some fun at the relative usefulness (or rather, uselessness) of chemical formulas for large organic molecules. While it is a useful concept for teaching people about chemistry and {{w|Chemical_equation#Balancing_chemical_equations|balancing equations}}, and it was useful in the early days of chemistry to try to categorize and learn about molecules via {{w|stoichiometry}} - it does not give much useful information. For example, even the simple formula C<sub>11</sub>H<sub>15</sub>NO<sub>2</sub> has 302 registered isomers.{{actual citation needed}} Many of them are NOT good to eat.{{cn}} | ||
| + | |||
| + | As is common practice for real compounds that contain organic structures or substructures, the numbers of atoms of carbon and hydrogen are listed before all of the others; the others are listed in alphabetical order. There are estimated to be 10<sup>80</sup> atoms of hydrogen (H), by far the most common element in the universe. The next most common element, helium (He), is a long way to the right in the list, and out of view, but would be about a third as many as the hydrogens. | ||
| + | |||
| + | In reality, there is not a fixed number of atoms of each element across the lifetime of the universe. The matter originally created in the {{w|Big Bang}} was unbound protons and neutrons. In the first few minutes, {{w|Big Bang nucleosynthesis|some of these combined to form lightweight nuclei}}, but most remained as {{w|proton}}s (i.e. the nuclei of hydrogen atoms). Other more complex atoms, up to {{w|atomic mass}} 56, formed later (and are still being formed) as a result of {{w|stellar nucleosynthesis}}. Still more massive nuclei have been and are being formed via {{w|supernova nucleosynthesis}}. Although the proportions of these atoms depend in a complex way on the fusion processes involved, and on the stabilities of those nuclei, the most massive atoms are generally both short-lived and less favored to form, so their elemental abundances in the universe are very small. As shown above, the number of americium (Am) atoms is much smaller than those of any other element in the visible part of the "formula". There are slightly fewer atoms of americium in the entire universe than the total number of atoms of hydrogen and oxygen in 1.0 L of liquid water. | ||
| + | |||
| + | The title text is probably referencing {{w|gravity}}, because, for the most part, these atoms would be "held together" only by gravity, and it is a very weak bond indeed. | ||
| + | |||
| + | The numbers of atoms are large, but they are not nameless. Using the {{w|long and short scales}}, these numbers can be described as: | ||
| + | {| class="wikitable sortable" | ||
| + | !Pos!!Symb!!Name !!Quantity !!Short Scale name !!Long Scale name(s) !!Ranked quantity* | ||
| + | |- | ||
| + | | 1|| C ||Carbon ||data-sort-value="1e76"|10<sup>76</sup> ||Ten quattuorvigintillion ||Ten thousand duodecillion<br/>Ten duodecilliard ||4 | ||
| + | |- | ||
| + | | 2|| H ||Hydrogen ||data-sort-value="1e80"|10<sup>80</sup> ||One hundred quinvigintillion ||One hundred tridecilllion ||1 | ||
| + | |- | ||
| + | | 3|| Ac ||Actinium ||data-sort-value="1e67"|10<sup>67</sup> ||Ten unvigintillion ||Ten undecillion ||data-sort-value="84"|≈84 | ||
| + | |- | ||
| + | | 4|| Ag ||Silver ||data-sort-value="1e69"|10<sup>69</sup> ||One duovigintillion ||One thousand undecillion<br/>One undecilliard ||data-sort-value="68"|≈68 | ||
| + | |- | ||
| + | | 5|| Al ||Aluminium<br/>Aluminum||data-sort-value="1e75"|10<sup>75</sup> ||One quattuorvigintillion ||One thousand duodecillion<br/>One duodecilliard ||14 | ||
| + | |- | ||
| + | | 6|| Am ||Americium ||data-sort-value="1e26"|10<sup>26</sup> ||One hundred septillion ||One hundred quadrillion ||data-sort-value="84"|≈84 | ||
| + | |- | ||
| + | | 7|| Ar ||Argon ||data-sort-value="1e75"|10<sup>75</sup> ||One quattuorvigintillion ||One thousand duodecillion<br/>One duodecilliard ||11 | ||
| + | |- | ||
| + | | 8|| As ||Arsenic ||data-sort-value="1e70"|10<sup>70</sup> ||Ten duovigintillion ||Ten thousand undecillion<br>Ten undecilliard ||data-sort-value="40"|≈40 | ||
| + | |- | ||
| + | | 9|| At ||Astatine ||data-sort-value="1e47"|10<sup>47</sup> ||One hundred quattuordecillion||One hundred thousand septillion<br/>One hundred septilliard ||data-sort-value="84"|≈84 | ||
| + | |- | ||
| + | | 10|| Au ||Gold ||data-sort-value="1e69"|10<sup>69</sup> ||One duovigintillion ||One thousand undecillion<br/>One undecilliard ||data-sort-value="68"|≈68 | ||
| + | |- | ||
| + | | 11|| B ||Boron ||data-sort-value="1e71"|10<sup>71</sup> ||One hundred duovigintillion ||One hundred thousand undecillion<br/>One hundred undecilliard||data-sort-value="61"|≈61 | ||
| + | |- | ||
| + | | 12|| Ba ||Barium ||data-sort-value="1e70"|10<sup>70</sup> ||Ten duovigintillion ||Ten thousand undecillion<br>Ten undecilliard ||data-sort-value="33"|≈33 | ||
| + | |- | ||
| + | | 13|| Be ||Beryllium ||data-sort-value="1e71"|10<sup>71</sup>*||One hundred duovigintillion ||One hundred thousand undecillion<br/>One hundred undecilliard||data-sort-value="61"|≈61 | ||
| + | |- | ||
| + | |data-sort-value="43"|43*||He||Helium||data-sort-value="3e79"|10<sup>79</sup>*||Ten quinvigintillion ||Ten tridecilllion ||2 | ||
| + | |- | ||
| + | |data-sort-value="73"|73*||O ||Oxygen||data-sort-value="1e78"|10<sup>78</sup>*||One quinvigintillion ||One tridecilllion ||3 | ||
| + | |} | ||
| + | :<nowiki>*</nowiki> - Information not provided by the comic; Source for ranked data, in particular, does not 'entirely' agree with the quantities that are given in the comic. | ||
| + | |||
| + | The formula as it appears in the comic is truncated. The complete formula of the universe in this style (but arranged in order of abundance after carbon) would be C₁₀⁷⁷ H₁₀⁸⁰ He₁₀⁷⁹ O₁₀⁷⁸ Ne₁₀⁷⁶ N₁₀⁷⁶ Mg₁₀⁷⁵ Si₁₀⁷⁵ Ar₁₀⁷⁵ Fe₁₀⁷⁶ S₁₀⁷⁶ Ni₁₀⁷⁵ Ca₁₀⁷⁵ Al₁₀⁷⁵ B₁₀⁷¹ Be₁₀⁷¹ Na₁₀⁷⁵ As₁₀⁷² Br₁₀⁷² Li₁₀⁷² Cr₁₀⁷⁵ Ti₁₀⁷⁴ Mn₁₀⁷⁴ P₁₀⁷⁴ K₁₀⁷⁴ V₁₀⁷⁴ Cl₁₀⁷⁴ F₁₀⁷³ Sc₁₀⁷² Co₁₀⁷⁴ Cu₁₀⁷² Zn₁₀⁷³ Ga₁₀⁷² Ge₁₀⁷³ Se₁₀⁷² Kr₁₀⁷² Rb₁₀⁷² Sr₁₀⁷² Y₁₀⁷¹ Zr₁₀⁷² Nb₁₀⁷¹ Mo₁₀⁷¹ Tc₁₀⁰ Ru₁₀⁷¹ Rh₁₀⁷⁰ Pd₁₀⁷¹ Ag₁₀⁷⁰ Cd₁₀⁷¹ In₁₀⁷⁰ Sn₁₀⁷¹ Sb₁₀⁷⁰ Te₁₀⁷² I₁₀⁷¹ Xe₁₀⁷² Cs₁₀⁷⁰ Ba₁₀⁷² La₁₀⁷¹ Ce₁₀⁷² Pr₁₀⁷¹ Nd₁₀⁷² Pm₁₀⁰ Sm₁₀⁷¹ Eu₁₀⁷⁰ Gd₁₀⁷¹ Tb₁₀⁷⁰ Dy₁₀⁷¹ Ho₁₀⁷⁰ Er₁₀⁷¹ Tm₁₀⁷⁰ Yb₁₀⁷¹ Lu₁₀⁷⁰ Hf₁₀⁷⁰ Ta₁₀⁷⁰ W₁₀⁷⁰ Re₁₀⁷⁰ Os₁₀⁷¹ Ir₁₀⁷¹ Pt₁₀⁷¹ Au₁₀⁷⁰ Hg₁₀⁷¹ Tl₁₀⁷⁰ Pb₁₀⁷² Bi₁₀⁷⁰ Po₁₀⁰ At₁₀⁰ Rn₁₀⁰ Fr₁₀⁰ Ra₁₀⁰ Ac₁₀⁰ Th₁₀⁷⁰ Pa₁₀⁰ U₁₀⁷⁰ Np₁₀⁰ Pu₁₀⁰ Am₁₀⁰ Cm₁₀⁰ Bk₁₀⁰ Cf₁₀⁰ Es₁₀⁰ Fm₁₀⁰ Md₁₀⁰ No₁₀⁰ Lr₁₀⁰ Rf₁₀⁰ Db₁₀⁰ Sg₁₀⁰ Bh₁₀⁰ Hs₁₀⁰ Mt₁₀⁰ Ds₁₀⁰ Rg₁₀⁰ Cn₁₀⁰ Nh₁₀⁰ Fl₁₀⁰ Mc₁₀⁰ Lv₁₀⁰ Ts₁₀⁰ Og₁₀⁰ according to estimates of abundance. | ||
==Transcript== | ==Transcript== | ||
| Line 21: | Line 67: | ||
{{comic discussion}}<noinclude> | {{comic discussion}}<noinclude> | ||
| + | [[Category:Chemistry]] | ||
| + | [[Category:Cosmology]] | ||
Latest revision as of 11:22, 29 January 2026
| Chemical Formula |
 Title text: Some of the atoms in the molecule are very weakly bound. |
Explanation[edit]
| This is one of 60 incomplete explanations: This page was created by the carbon in the universe . Don't remove this notice too soon. If you can fix this issue, edit the page! |
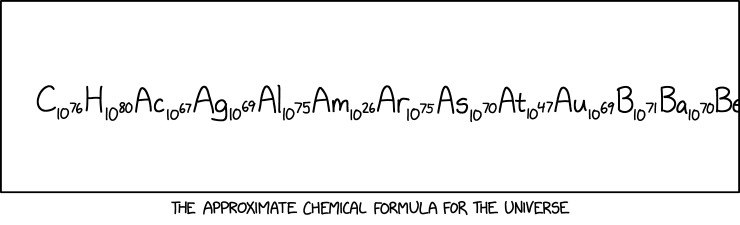
This supposed "chemical formula for the universe" merely lists the numbers of atoms of each element that are thought to exist in the observable universe. Usually, chemical formulae imply rather more of a discrete binding together of the atoms involved. They also represent a single molecule of the substance, rather than trying to cover the entire number of atoms in the whole quantity under consideration.
This may be poking some fun at the relative usefulness (or rather, uselessness) of chemical formulas for large organic molecules. While it is a useful concept for teaching people about chemistry and balancing equations, and it was useful in the early days of chemistry to try to categorize and learn about molecules via stoichiometry - it does not give much useful information. For example, even the simple formula C11H15NO2 has 302 registered isomers.[actual citation needed] Many of them are NOT good to eat.[citation needed]
As is common practice for real compounds that contain organic structures or substructures, the numbers of atoms of carbon and hydrogen are listed before all of the others; the others are listed in alphabetical order. There are estimated to be 1080 atoms of hydrogen (H), by far the most common element in the universe. The next most common element, helium (He), is a long way to the right in the list, and out of view, but would be about a third as many as the hydrogens.
In reality, there is not a fixed number of atoms of each element across the lifetime of the universe. The matter originally created in the Big Bang was unbound protons and neutrons. In the first few minutes, some of these combined to form lightweight nuclei, but most remained as protons (i.e. the nuclei of hydrogen atoms). Other more complex atoms, up to atomic mass 56, formed later (and are still being formed) as a result of stellar nucleosynthesis. Still more massive nuclei have been and are being formed via supernova nucleosynthesis. Although the proportions of these atoms depend in a complex way on the fusion processes involved, and on the stabilities of those nuclei, the most massive atoms are generally both short-lived and less favored to form, so their elemental abundances in the universe are very small. As shown above, the number of americium (Am) atoms is much smaller than those of any other element in the visible part of the "formula". There are slightly fewer atoms of americium in the entire universe than the total number of atoms of hydrogen and oxygen in 1.0 L of liquid water.
The title text is probably referencing gravity, because, for the most part, these atoms would be "held together" only by gravity, and it is a very weak bond indeed.
The numbers of atoms are large, but they are not nameless. Using the long and short scales, these numbers can be described as:
| Pos | Symb | Name | Quantity | Short Scale name | Long Scale name(s) | Ranked quantity* |
|---|---|---|---|---|---|---|
| 1 | C | Carbon | 1076 | Ten quattuorvigintillion | Ten thousand duodecillion Ten duodecilliard |
4 |
| 2 | H | Hydrogen | 1080 | One hundred quinvigintillion | One hundred tridecilllion | 1 |
| 3 | Ac | Actinium | 1067 | Ten unvigintillion | Ten undecillion | ≈84 |
| 4 | Ag | Silver | 1069 | One duovigintillion | One thousand undecillion One undecilliard |
≈68 |
| 5 | Al | Aluminium Aluminum |
1075 | One quattuorvigintillion | One thousand duodecillion One duodecilliard |
14 |
| 6 | Am | Americium | 1026 | One hundred septillion | One hundred quadrillion | ≈84 |
| 7 | Ar | Argon | 1075 | One quattuorvigintillion | One thousand duodecillion One duodecilliard |
11 |
| 8 | As | Arsenic | 1070 | Ten duovigintillion | Ten thousand undecillion Ten undecilliard |
≈40 |
| 9 | At | Astatine | 1047 | One hundred quattuordecillion | One hundred thousand septillion One hundred septilliard |
≈84 |
| 10 | Au | Gold | 1069 | One duovigintillion | One thousand undecillion One undecilliard |
≈68 |
| 11 | B | Boron | 1071 | One hundred duovigintillion | One hundred thousand undecillion One hundred undecilliard |
≈61 |
| 12 | Ba | Barium | 1070 | Ten duovigintillion | Ten thousand undecillion Ten undecilliard |
≈33 |
| 13 | Be | Beryllium | 1071* | One hundred duovigintillion | One hundred thousand undecillion One hundred undecilliard |
≈61 |
| 43* | He | Helium | 1079* | Ten quinvigintillion | Ten tridecilllion | 2 |
| 73* | O | Oxygen | 1078* | One quinvigintillion | One tridecilllion | 3 |
- * - Information not provided by the comic; Source for ranked data, in particular, does not 'entirely' agree with the quantities that are given in the comic.
The formula as it appears in the comic is truncated. The complete formula of the universe in this style (but arranged in order of abundance after carbon) would be C₁₀⁷⁷ H₁₀⁸⁰ He₁₀⁷⁹ O₁₀⁷⁸ Ne₁₀⁷⁶ N₁₀⁷⁶ Mg₁₀⁷⁵ Si₁₀⁷⁵ Ar₁₀⁷⁵ Fe₁₀⁷⁶ S₁₀⁷⁶ Ni₁₀⁷⁵ Ca₁₀⁷⁵ Al₁₀⁷⁵ B₁₀⁷¹ Be₁₀⁷¹ Na₁₀⁷⁵ As₁₀⁷² Br₁₀⁷² Li₁₀⁷² Cr₁₀⁷⁵ Ti₁₀⁷⁴ Mn₁₀⁷⁴ P₁₀⁷⁴ K₁₀⁷⁴ V₁₀⁷⁴ Cl₁₀⁷⁴ F₁₀⁷³ Sc₁₀⁷² Co₁₀⁷⁴ Cu₁₀⁷² Zn₁₀⁷³ Ga₁₀⁷² Ge₁₀⁷³ Se₁₀⁷² Kr₁₀⁷² Rb₁₀⁷² Sr₁₀⁷² Y₁₀⁷¹ Zr₁₀⁷² Nb₁₀⁷¹ Mo₁₀⁷¹ Tc₁₀⁰ Ru₁₀⁷¹ Rh₁₀⁷⁰ Pd₁₀⁷¹ Ag₁₀⁷⁰ Cd₁₀⁷¹ In₁₀⁷⁰ Sn₁₀⁷¹ Sb₁₀⁷⁰ Te₁₀⁷² I₁₀⁷¹ Xe₁₀⁷² Cs₁₀⁷⁰ Ba₁₀⁷² La₁₀⁷¹ Ce₁₀⁷² Pr₁₀⁷¹ Nd₁₀⁷² Pm₁₀⁰ Sm₁₀⁷¹ Eu₁₀⁷⁰ Gd₁₀⁷¹ Tb₁₀⁷⁰ Dy₁₀⁷¹ Ho₁₀⁷⁰ Er₁₀⁷¹ Tm₁₀⁷⁰ Yb₁₀⁷¹ Lu₁₀⁷⁰ Hf₁₀⁷⁰ Ta₁₀⁷⁰ W₁₀⁷⁰ Re₁₀⁷⁰ Os₁₀⁷¹ Ir₁₀⁷¹ Pt₁₀⁷¹ Au₁₀⁷⁰ Hg₁₀⁷¹ Tl₁₀⁷⁰ Pb₁₀⁷² Bi₁₀⁷⁰ Po₁₀⁰ At₁₀⁰ Rn₁₀⁰ Fr₁₀⁰ Ra₁₀⁰ Ac₁₀⁰ Th₁₀⁷⁰ Pa₁₀⁰ U₁₀⁷⁰ Np₁₀⁰ Pu₁₀⁰ Am₁₀⁰ Cm₁₀⁰ Bk₁₀⁰ Cf₁₀⁰ Es₁₀⁰ Fm₁₀⁰ Md₁₀⁰ No₁₀⁰ Lr₁₀⁰ Rf₁₀⁰ Db₁₀⁰ Sg₁₀⁰ Bh₁₀⁰ Hs₁₀⁰ Mt₁₀⁰ Ds₁₀⁰ Rg₁₀⁰ Cn₁₀⁰ Nh₁₀⁰ Fl₁₀⁰ Mc₁₀⁰ Lv₁₀⁰ Ts₁₀⁰ Og₁₀⁰ according to estimates of abundance.
Transcript[edit]
| This is one of 40 incomplete transcripts: Don't remove this notice too soon. If you can fix this issue, edit the page! |
- [A long panel with a chemical formula trailing off the right side]
- C1076 H1080 Ac1067 Ag1069 Al1075 Am1026 Ar1075 As1070 At1047 Au1069 B1071 Ba1070 Be
- [Caption below the panel:] The approximate chemical formula for the universe
Discussion
I'm disappointed that it wasn't scrollable. 2001:41D0:8:5062:0:0:0:1 20:20, 28 January 2026 (UTC)
- +1 And funny to think that the universe contains less than a few hundred mol of Americium. --2001:16B8:CC03:E100:8552:6543:7CF4:9AE7 20:57, 28 January 2026 (UTC)
- Time for a campaign to Make Americium Greater? 82.13.184.33 09:32, 29 January 2026 (UTC)
If anyone's interested in an accessible resource for getting more data like this, may I suggest https://ptable.com/#Properties/Abundance/Universe (which I believe derives data from IUPAC sources) Dextrous Fred (talk) 20:37, 28 January 2026 (UTC)
surprised to see so much Astatine, he himself declared, that stuff doesnt want to exist so I expected yet a few powers of ten less 2a00:6020:479f:6c00:d587:ac2a:d1e2:26a9 (talk) 21:08, 28 January 2026 (UTC) (please sign your comments with ~~~~)
This does make me curious: how would neutronium be represented in a chemical formula? Or would it be? My impression is it kind of exists 'outside' of chemistry... -Kalil 147.81.60.76 21:12, 28 January 2026 (UTC)
- Neutron stars would be represented with n with various mass numbers. And there are no more than 1 mmol (6.02214076×1020) of neutron stars. 2001:4C4E:1C09:EC00:7932:264E:A9E0:8ED0 21:38, 28 January 2026 (UTC)
What about adding mass numbers? For example, most of the hydrogen is 1H, with small amounts of 2H and trace amounts of 3H. 2001:4C4E:1C09:EC00:7932:264E:A9E0:8ED0 21:38, 28 January 2026 (UTC)
Oh look, it's the 3200th comic! Yay I guess! --DollarStoreBa'alConverse 22:46, 28 January 2026 (UTC)
An unregistered user (198.48.180.159) added a note that the chemical formula "C11H15NO2" (i.e. C11H15NO2) "has 302 registered isomers". I don't know the source for that number or where those isomers are registered. (It's the formula for MDMA, which is, as noted, "not good to eat".) Would that be the CAS registry? BunsenH (talk) 23:20, 28 January 2026 (UTC)
- Don't know if this works, but here's a site that does immediately return 302 compounds: https://pubchemlite.lcsb.uni.lu/compounds?query=C11H15NO2 8.17.60.225 04:19, 29 January 2026 (UTC)
10^26 atoms of americium is about 40 kg. But it looks like humans produced tons of americium: https://isis-online.org/uploads/isis-reports/documents/np_237_and_americium.pdf . If there are other civilizations in the observable Universe, then the amount of americium in the Universe is even higher. So I guess the formula counts only naturally produced elements. But even then it seems underestimated. Alexei Kopylov (talk) 23:45, 28 January 2026 (UTC)
- In everything that I've checked (I expanded the "list of names" into a table), I could not discover any universal quantity of americium that was close to Randall's apparent source. Can't exclude the possibility that artificially nucleogenesis played a part in his figures (while mine are from how much was created 'naturally'), but I've just had to go along with it being a completely wrong figure (for the ultimate universal ranking). Much as boron might be given slightly mismagnituded.
- However, if anyone thinks they have the same source that led to the comic's values (and can reconfirm beryllium's estimated order of magnitude, which is the only reason I decided to start on compiling this amount of extended data, which is actually for all 118 humanly known elements), then you're welcome to correct anything that I left in an incorrect state. 81.179.199.253 00:16, 29 January 2026 (UTC)
...but what if you had a mole of universes? 99.109.3.237 (talk) 00:50, 29 January 2026 (UTC) (please sign your comments with ~~~~)
In the explanation, towards the end of the formula for the universe, it says U₁₀². Would that mean that there are only about 100 uranium atoms in the whole universe? That seems way too low. Did the explainer confuse the powers of 10 with rankings (in reverse)? --208.59.176.206 03:48, 29 January 2026 (UTC)
- I'm not sure where the error came from, but about half the numbers are drastically too low. Remember, a mole is 6.02*10^23. 174.94.104.215 05:34, 29 January 2026 (UTC)
- Fixed. The powers were just in descending order, one by one. The current values reflect the actual amounts, give or take one or two orders of magnitude. --1234231587678 (talk) 06:04, 29 January 2026 (UTC)
In the first paragraph of the explanation it says that the number for helium would be about a third as the number for hydrogen. This seems to compare the total masses for both elements instead of the number of atoms. Hydrogen should account for aprox. 92% of the atoms while Helium is approx. 8%. 2A02:810D:9B99:7800:DECB:CADA:B418:2F1A 05:58, 29 January 2026 (UTC)
Re "Other more complex atoms, up to atomic mass 56, formed later ... as a result of stellar nucleosynthesis". Not all of them; there is another way. Boron and Beryllium are produced by cosmic ray spallation, the splitting of heavier atoms by the impact of energetic particles. 2A12:F43:141A:9F00:A0FA:9260:7BAF:8D57 13:16, 29 January 2026 (UTC) dww-uk