Difference between revisions of "2913: Periodic Table Regions"
Bugstomper (talk | contribs) (→Table Sections: add explanation for boron) |
(Added further clarification about the Lawful Neutral reference in the Noble Gases section.) |
||
| Line 48: | Line 48: | ||
| Very specific health problems || Iodine and radon || Iodine, Radon || Radon can accumulate in buildings, especially in basements and cellars, since it is a gas that is denser than air. Since it is also radioactive, this accumulation can then result in radiation sickness. Iodine is a required nutrient that humans need in trace amounts to remain healthy, with an iodine deficiency typically causing thyroid problems such as goitre. These two specific health problems are entirely unrelated and it is only by coincidence that they are next to each other on the periodic table. | | Very specific health problems || Iodine and radon || Iodine, Radon || Radon can accumulate in buildings, especially in basements and cellars, since it is a gas that is denser than air. Since it is also radioactive, this accumulation can then result in radiation sickness. Iodine is a required nutrient that humans need in trace amounts to remain healthy, with an iodine deficiency typically causing thyroid problems such as goitre. These two specific health problems are entirely unrelated and it is only by coincidence that they are next to each other on the periodic table. | ||
|- | |- | ||
| − | | Lawful Neutral || Noble Gases || Helium, Neon, Argon, Krypton, Xenon || These elements are mostly unreactive. | + | | Lawful Neutral || Noble Gases || Helium, Neon, Argon, Krypton, Xenon || These elements are mostly unreactive. Lawful Neutral is a reference to the D&D alignment chart, which gives moral categories for characters. The chart goes from Lawful to Chaotic on one axis, and Good to Evil on another. Lawful Neutral means following the law without any bias towards Good or Evil, which could be exemplified by the unreactivity of the Noble Gases. |
|- | |- | ||
| Don't bother learning their names – they're not staying long || Astatine and Period 7 from Rutherfordium onwards || Astatine, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium, Roentgenium, Copernicum, Nihonium, Flevorium, Moscovium, Livermorium, Tennessine, Oganesson || These elements are hard to produce in large quantities and most of them decay within hours. | | Don't bother learning their names – they're not staying long || Astatine and Period 7 from Rutherfordium onwards || Astatine, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium, Roentgenium, Copernicum, Nihonium, Flevorium, Moscovium, Livermorium, Tennessine, Oganesson || These elements are hard to produce in large quantities and most of them decay within hours. | ||
Revision as of 22:46, 29 March 2024
| Periodic Table Regions |
 Title text: Cesium-133, let it be. Cesium-134, let it be even more. |
Explanation
| This is one of 69 incomplete explanations: Created by a LAWFUL NEUTRAL MURDER WEAPON COMMONLY USED TO MAKE SPARK PLUGS' VOICES SQUEAKY- Please change this comment when editing this page. Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
The periodic table is used to arrange chemical elements based on their properties. This comic groups them together into regions.
Table Sections
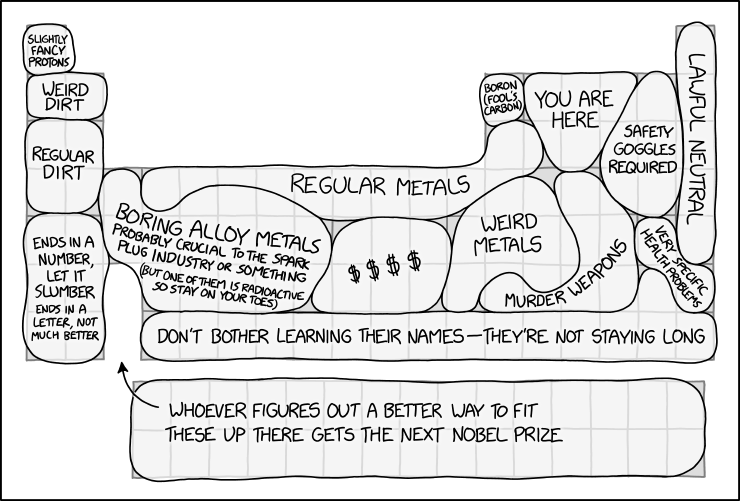
| Section | Real table | Elements contained | Explanation |
|---|---|---|---|
| Slightly fancy protons | Hydrogen | Hydrogen | Hydrogen atoms are a proton and an electron. Since the electron can be removed and you can call that a Hydrogen+ ion, hydrogen is a slightly fancy proton. |
| Weird dirt | Group 1 and 2 metals | Lithium, Beryllium | Lithium and beryllium, as some of the lightest elements, have unusual properties compared to heavier metals. Lithium, for instance, is the least dense metal on the periodic table, and beryllium is both toxic and transparent to x-rays. |
| Regular dirt | Group 1 and 2 metals | Sodium, Magnesium, Potassium, Calcium | Despite being metals, these are listed as "dirt" rather than "metal." Perhaps this is because they are commonly found in dirt, as they are essential nutrients for plant life (and all other life forms). |
| Ends in a number, let it slumber. Ends in a letter, not much better. | Group 1 and 2 metals | Rubidium, Strontium, Cesium, Barium, Francium, Radium | Highly reactive metals, some of which are commonly used as radioactive isotopes (which are known by a number). |
| Boring alloy metals. Probably crucial to the spark plug industry or something. (But one of them is radioactive so stay on your toes.) | The left transition metals | Scandium, Vanadium, Chromium, Manganese, Yttrium, Zirconium, Niobium, Molybdeneum, Technetium, Ruthenium, Hafnium, Tantalum, Tungsten, Rhenium | Not actually so boring, but they tend to be used as consituents (sometimes as a small but vital trace) in alloys with specific uses, including stainless steel, bulb filaments and superconductors. A spark plug may use austenitic stainless steel, which includes chromium and (in some cases) molybdenum, for heat and oxidation resistance. Technetium is the lightest element that has no stable isotope. |
| Regular metals | The top transition metals | Titanium, Manganese, Iron, Cobalt, Nickel, Copper, Zinc, Aluminum, Silicon | Commonly known metals (and one metalloid, silicon). These all have important uses in construction and other major industries. |
| $$$ | The platinum group | Rhodium, Palladium, Silver, Iridium, Platinum, Gold | Rare and highly prized metals. |
| Weird metals | The "ordinary metals" and some transition metals | Gallium, Germanium, Cadmium, Indium, Tin, Mercury | These are more obscure than the other metals (except tin and mercury) and tend to have fewer or more specialized uses. Mercury is also the only metal that is liquid at room temperature, and gallium melts just above that at 30 °C (86 °F). |
| Boron (fool's carbon) | Boron | Boron | A search for "carbon" in boron finds mention of a number of similarities between the two elements. "Fool's carbon" is analogous to "fool's gold". |
| You are here | Nonmetals | Carbon, Nitrogen, Oxygen, Phosphorus | Elements involved in biological processes |
| Murder weapons | Ordinary metals and metalloids | Arsenic, Antimony, Tellurium, Thallium, Lead, Bismuth, Polonium | Arsenic, thallium, lead, and polonium are highly toxic and have been involved in many notorious poisoning cases. Antimony and tellurium are also hazardous, though to a lesser degree. Bismuth is the odd one out, having little toxicity at all, but it is used in lead free bullets and shot; the compound bismuth subsalicylate is the main ingredient in Pepto-Bismol. |
| Safety Goggles required | The halogens | Fluorine, Sulfur, Chlorine, Selenium, Bromine | These elements are highly reactive. |
| Very specific health problems | Iodine and radon | Iodine, Radon | Radon can accumulate in buildings, especially in basements and cellars, since it is a gas that is denser than air. Since it is also radioactive, this accumulation can then result in radiation sickness. Iodine is a required nutrient that humans need in trace amounts to remain healthy, with an iodine deficiency typically causing thyroid problems such as goitre. These two specific health problems are entirely unrelated and it is only by coincidence that they are next to each other on the periodic table. |
| Lawful Neutral | Noble Gases | Helium, Neon, Argon, Krypton, Xenon | These elements are mostly unreactive. Lawful Neutral is a reference to the D&D alignment chart, which gives moral categories for characters. The chart goes from Lawful to Chaotic on one axis, and Good to Evil on another. Lawful Neutral means following the law without any bias towards Good or Evil, which could be exemplified by the unreactivity of the Noble Gases. |
| Don't bother learning their names – they're not staying long | Astatine and Period 7 from Rutherfordium onwards | Astatine, Rutherfordium, Dubnium, Seaborgium, Bohrium, Hassium, Meitnerium, Darmstadtium, Roentgenium, Copernicum, Nihonium, Flevorium, Moscovium, Livermorium, Tennessine, Oganesson | These elements are hard to produce in large quantities and most of them decay within hours. |
| Whoever figures out a better way to fit these up there gets the next Nobel Prize | The internal transition metals | Lanthanum, Cerium, Praesodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, Lutetium, Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium | The lanthanides and actinides are placed kinda awkwardly "outside" of the main periodic table; alternative ways of displaying the elements, such as just putting them between the alkali earth elements and the transition metals, are usually considered visually less appealing. |
Transcript
| This is one of 44 incomplete transcripts: Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
- [A periodic table with regions labeled.]
- [Hydrogen:]
- Slightly fancy protons
- [Lithium and beryllium:]
- Weird dirt
- [4 elements below:]
- Regular dirt
- [6 elements further below:]
- Ends in a number, let it slumber
- ends in a letter, not much better
- [Left side of the transition metals group:]
- Boring alloy metals
- Probably critical to the spark plug industry or something
- (but one of them is radioactive so stay on your toes)
- [Most of the top row of the transition metals + aluminum:]
- Regular metals
- [Below the rightmost "regular metals":]
- Weird metals
- [Between "boring alloy metals" and "weird metals":]
- $$$$
- [Boron:]
- Boron (fool's carbon)
- [Top-center of p-block:]
- You are here
- [Top-right of p-block, excluding the rightmost column:]
- Safety goggles required
- [5 uppermost elements of the rightmost column:]
- Lawful neutral
- [Iodine and radon:]
- Very specific health problems
- [Below and to the right of "weird metals":]
- Murder weapons
- [Bottom row from the fourth column onwards:]
- Don't bother learning their names - they're not staying long
- [The lanthanides and actinides below the rest of the table, arrow pointing to a gap in the third column:]
- Whoever figures out a better way to fit these up there gets the next Nobel Prize
Discussion
Working on the table catalog. For ones that are part of multiple groups, I used the one that takes up the most. For split ones like Mn, I put them both. For Cs, please don't change it because Randall's American and that's how he would think of it. I know what all the elements are so please don't edit conflict me. --Purah #126 (talk) 20:50, 29 March 2024 (UTC)
- I'm done. Again, please don't have an edit war over cesium and aluminum. --Purah #126 (talk) 20:50, 29 March 2024 (UTC)
- What about Hahnium? ;) 172.70.163.31 20:54, 29 March 2024 (UTC)
- That's only for some people in Berkeley, if someone can find evidence that he's used it before in a comic then sure lol. --Purah #126 (talk) 21:12, 29 March 2024 (UTC)
- At times, dubnium, hassium, meitnerium and darmstadtium were considered for naming after Otto Hahn, and it reached several stages of international deliberation.
- I actually learnt my chemistry in a (UK) lab with a wall-poster periodic table that had Hahnium shown on it. But I can't quite remember in what position, nor can I remember enough of the other differences (there being several) from the actual 'official' namings, so cannot be sure exactly what provenance it had. Pre '92, though, which rules out some of the options.
- ...but memorable enough for me to 'keep an eye out' for the fate of the name in later years. 172.69.43.243 21:56, 29 March 2024 (UTC)
- Being a Nazi ended it for him. Russians don't like Nazis and wouldn't allow the name to go through. Meitnerium was a carefully chosen slap in the face. I wouldn't count on Hahnium happening any time soon.172.70.115.185 14:12, 31 March 2024 (UTC)
- That's only for some people in Berkeley, if someone can find evidence that he's used it before in a comic then sure lol. --Purah #126 (talk) 21:12, 29 March 2024 (UTC)
- What about Hahnium? ;) 172.70.163.31 20:54, 29 March 2024 (UTC)
What about the two categories implied by "Ends in a number, let it slumber. Ends in a letter, not much better"? Which elements are in which? -- Dtgriscom (talk) 01:36, 30 March 2024 (UTC)
- I'd say "all in both". Any given "fooium" is going to be a reactive element that (especially as you go further down) is nasty to deal with 'raw'. But not as nasty as "fooium-123", which highly suggests a nuclear decay product that will further decay. 172.71.178.218 02:39, 30 March 2024 (UTC)
Correcting some errors in the stuff about radon led me to add perhaps too much information about how it's formed, how it accumulates in basements, and how it causes health problems. I spent a few years in a lab whose primary research focus was on uranium mine waste and its consequences, so I've got some bias and blind spots. Others may want to edit it down. BunsenH (talk) 01:39, 30 March 2024 (UTC)
- FWIW, the idea that radon collects in basements because of its density is a myth. It mixes with air; any variation with altitude is negligible, just as xenon and CFCs don't collect at low altitudes. BunsenH (talk) 04:42, 30 March 2024 (UTC)
I'm too lazy to add this myself, but should there be something in the explanation about the overlap between "weird metals" and "murder weapons? (I checked over it quickly, correct me if there is already something about it) EDIT - just realized this could be due to mercury being in "murder weapons" - Thexkcdnerd 02:02, 30 March 2024 (UTC)
There should be a comment on the fact that people have included that last row inside the table, [such as here](https://en.wikipedia.org/wiki/Extended_periodic_table#Predicted_structures_of_an_extended_periodic_table). It makes it really long, but there's a pretty basic pattern you can extend endlessly. Every other row, you lengthen it by four more than the previous pairs of rows. Though I understand the way orbitals work that make that useful doesn't really apply after you get too far down. DanielLC (talk) 03:23, 30 March 2024 (UTC)
- That has been done (not by me), now. But I also added (along the way) something about how not all PTs actually shift La and Ac out of the table. Though, honestly, I actually find it darn unusual (in my experience) to do that, often with those two under Sc and Y, and then the 'floating' groups go from up to Lu and Lr, perhaps starting with a 'repeat hint' of the eponymous elements, then Ce and Th. Otherwise, a split indicated between Group 2 and 3 and the two 14-long sets (La to Yb and Ac to No) with Lu and Lr group-3ed (more accurate to f-block?).
- Actually just overturned a few piles of books to try to find my old (30+ years!) degree-level material to confirm what exactly I recall working with back then, but they're probably stashed away elsewhere. Contemporary online versions do all kinds of things, sometimes seemingly according to abstract whims and clearly there's still no 'official' concensus... Hence I left it at "not all" doing so. 172.70.85.27 09:33, 30 March 2024 (UTC)
- I mean, "Lanthanide" means "Lanthanum-like". Is lanthanum like itself? It either very definitely is, pretty much pefectly,[citation needed] or it really isn't like it, for the same reason... (But, whatever the answer, we should probably say that actinide is the opposite for the actinides. To satisfy everyone!) 172.70.85.141 12:39, 30 March 2024 (UTC)
There should be a map with the elements under the bubbles Moderator (talk) 16:42, 30 March 2024 (UTC)
Currently Titanium appears twice in the table. Sebastian --172.68.110.60 20:36, 30 March 2024 (UTC)
- Fixed. Some of the elements are split between "groups", but titanium is solidly in the "regular metals". BunsenH (talk) 15:50, 31 March 2024 (UTC)
- Okay, there seems to be some disagreement on this one. The titanium box (4th row, 4th column) is almost entirely in the "regular metals" region; in fact, the region swells up for titanium so as to capture most of that box. Should it be listed in both that region and the "boring alloy metals" region? I don't think so, but ...? My inclination is to have it in only one group, which is obviously "regular metals". BunsenH (talk) 19:59, 31 March 2024 (UTC)
I had a bit of time to spare, and I was considering the following either as a reworking of the table or an "indexing" table (make all xkcd main/overlap class names link to the appropriate internal section-headers, so we don't need to squash them up into a narrow column, and can add a "complete list" of elements to as a closing paragraph if that seems best) ...
| Big table | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
...some notes:
- I did play with rowspanning, but my added sortability didn't make it worthwhile. It breaks the rowspanning then doesn't remake them, upon sorting. And doesn't make a span out of things sorted into connection.
- I also tried a few things out in Common Class. It all started off as Group id (except for Lanths/Acts), then I started going fancy.
- There are arguably some classifications that work well or against the Munrovian definition, interesting in both cases.
- Maybe I should have done a "Transition metal (special subclass)" type of thing, or stuck with "x-block" variations, or something with the standard metal/non-metal/semimetal thing.
- There's also the option of background colouring the lines by some specification or other. But I couldn't decide what scheme to use that for.
- For "main" xkcd classification, I gave whatever (seemed to) cover most of the element tile. For "overlap" I gave only those secondary classifications that did more than apparently accidentally stray over the line (e.g. on the way to/from covering other tiles, diagonally away from where they most obviously applied).
- I didn't bother to check that every classification I assigned was true (and/or intentionally funny, of course). Nor if it matched the existing table's assumptions.
- Where there's (common/notable) alternate forms of things I ()ed the form that I don't think Randall uses, but others certainly do (or have). I'm personally a Sulphur/Aluminium person, myself, e.g., and my unasked-for spellcheck really doesn't like the alternatives, but I'm not a Wolfram one.
- As I was making sure the Group column sorted nicely, I sneakily gave the Lanths/Acts 'pseudo group' numbers to perhaps keep them/restore them to the correct relative position without necessarily relying upon an Atomic Number sort. You can also do anything you like with the non-sort display item for that column ("Group IIb"/etc), that way.
Anyway, now it's here, if anyone wants to do anything more with it. 162.158.74.24 00:40, 31 March 2024 (UTC)
Can we all agree that silicon got done dirty? 172.70.231.87 18:12, 1 April 2024 (UTC)
I initially read the 'don't bother learning them' line as saying that these elements are "hard to pronounce in large quantities". Which, funnily enough, isn't far wrong.172.69.194.121 09:04, 2 April 2024 (UTC)
- It helps if one sets them to music. :-) BunsenH (talk) 16:02, 2 April 2024 (UTC)
 Add comment
Add comment
