Difference between revisions of "2975: Classical Periodic Table"
(don't dance around what elements they refer to) |
|||
| Line 41: | Line 41: | ||
[The regions and colors are as follows: | [The regions and colors are as follows: | ||
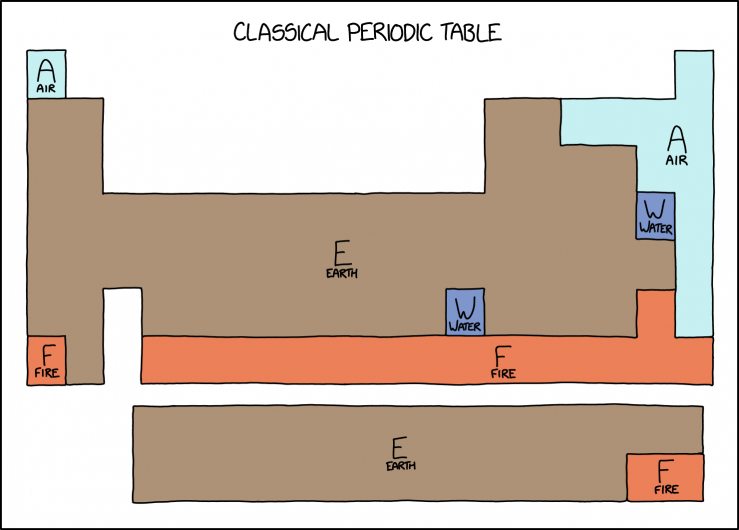
| − | * A: Air: light blue: | + | * A: Air: light blue: Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, and the noble gases. |
| − | * W: Water: dark blue: | + | * W: Water: dark blue: Bromine and Mercury. |
| − | * F: Fire: red-orange: | + | * F: Fire: red-orange: Astatine, Francium, and everything from Mendelevium onwards. |
* E: Earth: brown: everything else, the majority of the diagram. | * E: Earth: brown: everything else, the majority of the diagram. | ||
] | ] | ||
{{comic discussion}} | {{comic discussion}} | ||
Revision as of 20:08, 21 August 2024
| Classical Periodic Table |
 Title text: Personally I think mercury is more of a 'wet earth' hybrid element. |
Explanation
| This is one of 63 incomplete explanations: Created by THE AVATAR - Please change this comment when editing this page. Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
An element is a basic atomic building block of the physical world. Ancient civilizations believed in a small number of broad elements. The most famous are the classical (Hellenistic) elements of earth, fire, air, water, and sometimes a fifth element such as "void", "ether", or "quintessence." The Chinese wuxing system was a bit different, dropping air and adding elements for wood and metal. Such elemental theories fell out of favor as alchemists and later scientists began to discover what we now recognize as the atomic model, and today 118 elements are recognized and organized into the Periodic Table of Elements.
Here, Randall has taken a modern periodic table and color-coded the modern elements with the four classical elements. Gaseous elements such as hydrogen are colored blue for "air." Bromine and mercury, the two elements that remain liquid at room temperature and pressure, are colored dark blue for "water." Radioactive elements along the bottom of the table whose isotopes have only extremely short half-lives are red for "fire," with the rest of the chart filled in brown for "earth." The "earth" region includes many elements which are radioactive but whose half-lives are greater than 1 day.
The title text says that mercury should be classified as "wet earth". While it's a liquid, it has a very high surface tension so even large drops will stick together and may seem almost like a gel.
Table Sections
| Section | Real table | Elements contained | Explanation |
|---|---|---|---|
| Air | All noble gases and most reactive nonmetals | Hydrogen, Nitrogen, Oxygen, Fluorine, Neon, Helium, Chlorine, Argon, Krypton, Xenon, Radon | These elements are a gas at room temperature, so they are grouped into "Air". |
| Water | One metal and one nonmetal | Bromine, Mercury | These elements are liquid at room temperature, so they are grouped into “Water”. |
| Fire | One alkali metal and many synthetic metals | These are all highly radioactive metals with a half-life less than one day and a tendency to violently decompose at standard temperature and pressure, hence they are classified as "Fire". |
This comic is similar to 2913: Periodic Table Regions, which also groups elements using unconventional methods.
Transcript
| This is one of 42 incomplete transcripts: Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
[Title text: Classical Periodic Table]
[A diagram in the shape of the periodic table of elements is split into labeled colored regions. Each region is labeled with a large letter over a word in smaller letters.]
[The regions and colors are as follows:
- A: Air: light blue: Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, and the noble gases.
- W: Water: dark blue: Bromine and Mercury.
- F: Fire: red-orange: Astatine, Francium, and everything from Mendelevium onwards.
- E: Earth: brown: everything else, the majority of the diagram.
]
Discussion
I checked the Actinides and it looks like the criteria for "fire" is half-life < 1 day. SpriteGuard (talk) 18:11, 21 August 2024 (UTC)
- I would have found it funnier, if only on table-esthetic grounds, if all radioactive elements had been filed as Fire. (I'm a chemist.) 198.41.242.175 07:58, 22 August 2024 (UTC)
The four classical Elements are still recognised by scientists; They just repurposed the word "element", and so have adopted "state" to describe this older classification - Solid, Liquid, Gas and Plasma exactly map to the classical 'elements'. I think we can forgive the medieval alchemical community for not recognising Bose-Einstein Condensate as their fifth element. 172.68.64.149 21:05, 21 August 2024 (UTC)
- what about non-newtonian fluids? 42.book.addict (talk) 22:17, 21 August 2024 (UTC)
- They're fluids... turns out. 172.70.126.229 04:47, 22 August 2024 (UTC)
- If the 2016 movie "Spectral" is to be believed, then Bose–Einstein condensates maps to ghosts. 172.68.0.167 23:26, 21 August 2024 (UTC)
Everyone knows that the 5th element is Leeloo Dallas. Obviously the Bose-Einstein Condensate would have to be the 6th Element.172.70.111.60 15:00, 22 August 2024 (UTC)
- That agrees with the comment (currently) above. If you can sense one of those, you can probably also see dead people... 172.70.90.207 20:00, 22 August 2024 (UTC)
He's done this comparison between 'classical' and 'modern' elements before... for example in comic #965. 172.70.58.3 05:52, 22 August 2024 (UTC)
I did what I could for the Fire section earlier, and I've just gone back and added some relevant What-If context for both Mercury and Astatine. The latter is nasty stuff - lucky it can't really exist under standard temperature and pressure, or we'd all be screwed. Darkwolf218 (talk) 08:45, 22 August 2024 (UTC)
- The issue of STP doesn't really apply to the whole "fire" grouping, anyway. The thing making them "fire" is not really influenced by changes in temperature and pressure (not unless you're going the way of stellar temperatures and being pressured by an essentially neutronium soup, but we're drifting into many more things being dragged into this, ultimately). The likes of phosphorous is not shown as "fire", when it can be indeed aflame under STP (to not be, you have to ensure the pressure is not from our typical mix of "air", but one which is quite a bit less reactive), and I'm really not sure (without looking up likely examples) how many of the non-"fire" elements (other than itself, of course) would not effectively set on fire if the pressure requirements was to be provided by a fluorine atmosphere...
- But any concept of "standard temperature, pressure and environment" is obviously beyond the scope of this humorous take. One could even imagine that it be "an infinite space" of the target (nonclassical) element (c.f. "infinite plane" assumptions), without boundary or container, but how that effects the fire/earth boundary of certain radioactive materials (w.r.t. the ability to have a critical density, given a supercritical mass without being forced together) I think is not necessarily open (or easily obtained) information. 172.70.160.220 12:17, 22 August 2024 (UTC)
- Phosphorous combines with other elements, like oxygen, to make fire. Radioactive elements produce heat and sometimes higher-frequency light (i.e. "fire") all by themselves. STP with neon as the gas might be a good starting point to think about this, although I wouldn't bet against neon interacting at least a little with some of the more aggressive fire elements. 162.158.155.191 19:40, 23 August 2024 (UTC)
- To be most sure (given fluorine's actual reactiveness with elements that have more stealth 'shell slots' available to attach to), I'd suggest helium over any other choice. Assuming you have it or can get it, naturally.
- Though, as pointed out, the tendencies of radioactivity really aren't subject to temperature or (short of metals being compressed to super-criticality in nuclear-weapons, which isn't really the same thing) pressure in any way. These do dictate the precise memberships of the non-Fire elements (you can freeze mercury or melt sodium or condense nitrogen, etc), but talking of STP isn't really so necessary in the context of the 'firey fringes', assuming it's based upon arbitrarily-timed half-life-length. 172.70.162.163 21:34, 23 August 2024 (UTC)
I feel like Munroe just discovered the Aristotle Version of Tom Lehrer's song The Elements 172.70.80.229 (talk) 16:52, 4 September 2024 (please sign your comments with ~~~~)
Ironically, under this periodic table, H2O would be considered 100% pure earth 172.68.35.59 (talk) 16:52, 1 October 2024 (please sign your comments with ~~~~)
Who remembers the 21st night of September? 162.158.79.173 20:29, 18 November 2024 (UTC)
in some cultures there's also "metal" --me, hi (talk) 19:29, 28 March 2025 (UTC)