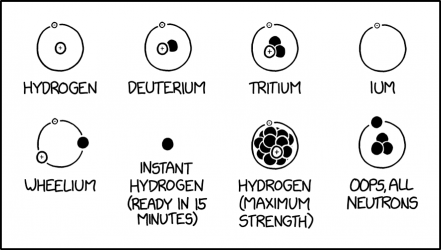
2719: Hydrogen Isotopes
| Hydrogen Isotopes |
 Title text: Oops, All Neutrons is also known as Neutral Quadrium, Nydnonen, and Goth Tritium. |
Explanation
| This is one of 72 incomplete explanations: Created by a BREAK ROOM DE BROGLIE MICROWAVE USER. Do NOT delete this tag too soon. If you can fix this issue, edit the page! |
Hydrogen is the simplest of the chemical atoms, usually consisting of an electron orbiting a lone proton, but with two other naturally occurring isotopes. This comic shows real and humorously fictional forms of hydrogen, generally depicted according the Chadwick model of the atom; see 2100: Models of the Atom for details.
| "Isotope" | Real? | Description |
|---|---|---|
| Hydrogen | Yes | Hydrogen-1 is the most common isotope of hydrogen, with one proton and one electron, shown with the electron orbiting the proton. It is also known as protium. |
| Deuterium | Yes | Deuterium is the second most common isotope of hydrogen, with one electron and both a neutron and proton in its nucleus. About one of every 6,760 hydrogen atoms in seawater is deuterium. Its chemical symbol is D or 2H, and it's also called heavy hydrogen or hydrogen-2. |
| Tritium | Yes | Tritium is the third most common isotope of hydrogen, with an electron orbiting a nucleus of one proton and two neutrons, for an atomic mass of about three daltons. It is radioactive with a half-life of about twelve years, and is very rare (but not as rare as unbound "instant hydrogen" neutrons.) It's also designated hydrogen-3, with the symbol of T or more often, 3H. |
| Ium | Only in the lab | This is a free electron orbiting around nothing. In line with the naming of the heavier hydrogen isotopes (a prefix designating the number of nucleons is followed by the suffix "-ium"), the lack of a nucleus is designated here by the absence of a prefix. A free electron will not circle around nothing but will react to electromagnetic fields when suitably configured. A Penning trap can confine electrons to move in circles. |
| Wheelium | No | This fictional form consists of a proton, electron, and neutron orbiting around nothing, shaped similarly to a wheel. The neutron could bind to the proton, but will more likely elastically scatter away. |
| Instant hydrogen (ready in 15 minutes) | Yes, but rare[1] | This is just a single neutron. An unbound neutron will decay into a proton, an electron, and an antineutrino, with a mean lifetime of just under fifteen minutes. The proton and electron can form into a hydrogen atom, but that only happens about four times in a million. The name is likely a reference to "instant" food such as noodles which are reduced for convenience and can be quickly reconstituted. |
| Hydrogen (maximum strength) | No | This fictional isotope consists of a proton, an electron, and what appear to be at least 14 neutrons. This isotope's proton would not be bound to all the neutrons. It would immediately decay by dripping most all of them away, producing a large amount of energy. "Maximum strength" may be a reference to over-the-counter medicines containing the largest quantity of active ingredients permitted. |
| Oops, All Neutrons | Extremely unlikely | This fictional form consists of four neutrons, with one orbiting around a group of three. As the existence of tetraneutrons is still uncertain, their possible configurations are unknown. But the depicted configuration is very unlikely given the characteristics of fundamental forces. The name is likely a reference to an American breakfast cereal called Oops! All Berries.|}
The title text provides three other names: 1. "Neutral Quadrium": Quadrium is the extremely rare artificial isotope hydrogen-4, with a proton and three neutrons.[2][3] The proton and electron have been replaced with neutrons. 2. "Nydnonen" is the word "hydrogen" with three consonants replaced by the letter 'n' so it has four of them representing the four neutrons. 3. "Goth Tritium": All the particles in the depiction are black, resembling typical gothic fashion, and in the same configuration as the particles of tritium. Transcript
DiscussionThis shows as a 404 on xkcd.com but in my RSS feed i can see the comic
Could someone add an explanation of Nydnonen? I don't get it and it's google proof 172.71.210.209 05:04, 3 January 2023 (UTC)Benzodiakanine
Are these to scale? I recently read that the Helium is smaller in terms of measured atomic radius than the Hydrogen. Possibly this is true of Deuterium as well? 172.70.85.45 06:50, 3 January 2023 (UTC)
Is "oops all neutrons" distinct from Neutronium, which is also all neutrons? 172.70.100.131 07:38, 3 January 2023 (UTC)
I think "Maximum Strength" is a reference to medicines marketed as such - in particular brands of Ibuprofen "Maximum Strength Tablets". --172.69.79.132 14:59, 3 January 2023 (UTC)
Considering that Deuterium is derived from Greek and Tritium works in both Greek and Latin, wouldn't the correct name for ⁴H be Tetartium?
Is it just me or have the recaptchas gotten much more difficult over the past week, to the point of ambiguous or indiscernibly blurred images and frequently rejecting correct responses (i.e. "please try again" in red)? Granted, I'm not saying this behavior makes it any less valid as a captcha, but it's a little surprising to always get several-step challenges lately. 172.71.154.38 05:08, 4 January 2023 (UTC)
Re "ium": Shouldn't we try to keep the explanation short and to the point? This comic is about "isotopes", i.e. about different options of how to construct a single atom (or atom-like entity). IMO, there is no need to include many-body effects in a set of multiple electrons ("Fermi velocity" or "electron degeneracy pressure"); just as there is no need to discuss, say, the kinetic theory of gases made up of these isotopes, or how they would be able to form fluids or solids. It is good to see that people who contribute here know about these effects, but I think that the explanation does not benefit from extending the discussion too far beyond the subject of a given comic. If anything, it might be worthwhile to include a reference to ion traps - especially since in a Penning trap electrons actually go in circulating orbits (although not exactly circular). --172.70.246.210 11:56, 4 January 2023 (UTC)
I love the administratium joke, but adding more jokes in the description seems antithetical to the purpose of this website :) 172.70.100.107 21:30, 4 January 2023 (UTC)
One thing about this cartoon strikes me as impressive. "Hydrogen, maximum strength" is about 5-10 % protons. Bulk nuclear matter, which is what makes up most of the body of a neutron star, has a neutron/proton ratio between 10 and 20 (probably close to the higher value). Hydrogen (maximum strength) is what holds up a neutron star, and that qualifies as pretty strong in anybody's book. And, while on the topic, there is no such thing as "neutronium". The cores of neutron stars are covered by a "nilium ocean" of free neutrons - but that term applies to bulk properties of the ocean. Go to the microscopic picture, and its just free neutrons; it is not a substance in its own right. A second point, if I may. An electron can orbit an empty place quite easily if you put it in a magnetic field - but there is another possibility. Dark matter exerts gravitational attraction on electrons. Given enough room, an electron will orbit dark matter. Perhaps "ium" should be called "darkium". -- Adeblanc (talk) 14:57, 2 February 2023 (please sign your comments with ~~~~)
What if we made a Rubiks Cube out of "Oops, All Neutrons"? That would be fun. (We would probably all die) Psychoticpotato (talk) 21:30, 4 April 2024 (UTC)
|
